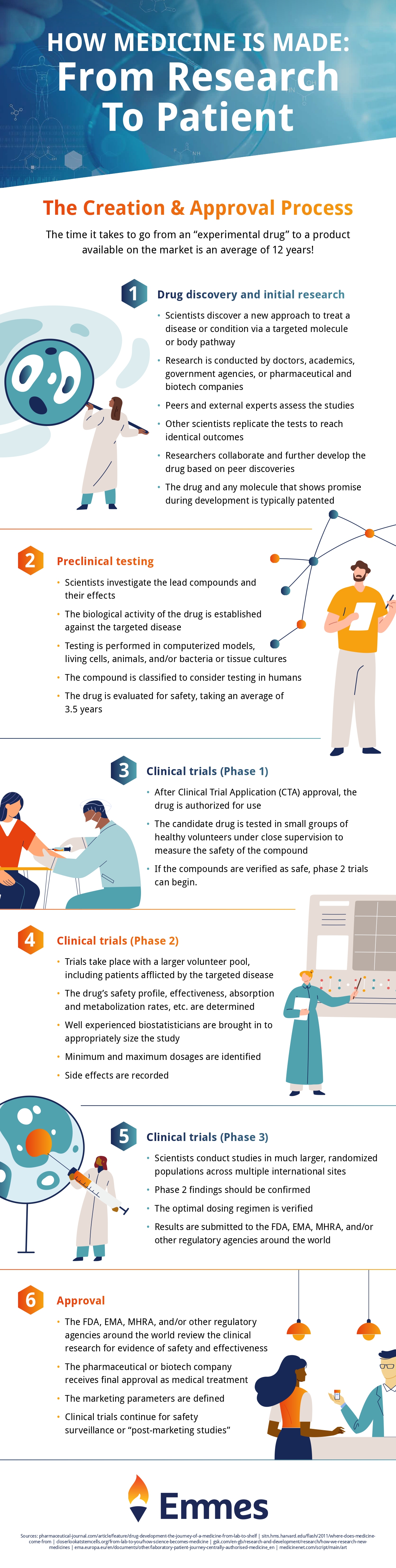
Drug development is always time-consuming and costly and preclinical development studies are always important which helps you make the proper studies in the functioning of the drugs before it comes into the market. It is a long term process in the development of drug and it takes almost seven years period in practice but sometimes it will take twelve years to complete the process. Lots of chemical compounds can be mixed to prepare the potential medicine and sometimes some of the chemical compounds can be eliminated in the process of developing a proper drug which will help to save the lives of millions of people across the globe.
Drugs which are manufactured with different chemical compounds will be tested in clinical trials with animals and if it found success then it will be brought back to the market. During this long-standing many drugs fails to become a proper medicine and can be neglected during the clinical studies. Here is the list of different phases involved in the process of developing a drug:
Drug discovery:
The first stage of drug development involves in identifying the target which means for what disease you are going to find drug. Once the target is identified then screening tools and computer databases will be used to find the chemical compounds that can be effective to find out a drug for the particular disease. If a specific compound is found to affect the target then it will be considered as a drug candidate. Out of 10,000 hits, the only one drug will be discovered and it will reach the market.
Drug trial:
After the drug discovery, the drug will get injected into animals to check the toxicity and performance. The animals can be in the form of a rat, dog, and macaque. After passing the toxicity now the research will be done for early formulations to make into tablet, capsule or injection. Then the preclinical results will be submitted to concerned authorities for approval.
Human trial:
Initially, it will be injected to the healthy people and at initial stages only single-doses will be given to them to check whether it brings any side effects or not. Usually, a small group of 5 to 10 members will be given a single dose and can be carefully monitored. This will help them to identify the poisonous substance and the doses can be incremented to multi-dose for 1-2 weeks. Then doses will be given to a slightly larger group of peoples and are carefully monitored.
Dose-finding studies:
After the successful testing with smaller sections now the drug will be tested with a large pool of peoples around 800-1000 peoples. Safety of the people will be monitored by the drug safety monitoring board. Data will be taken properly and maintained and sometimes it will be submitted to regulatory agencies for the approval. Chances for drug not get approved even in this stage.
Follow-up studies:
After getting approval from the authorities the drug will be given to the people continuously for a longer period of time. This is done to monitor the side effects and other safety concerns. If the safety commitments are not followed then the drug will get rejected.
Pre-clinical research is just one stage in the medicine making process. Check out the infographic below to see how your medicine is made, from start to finish!
Infographic provided by The Emmes Company, a clinical data management organization